
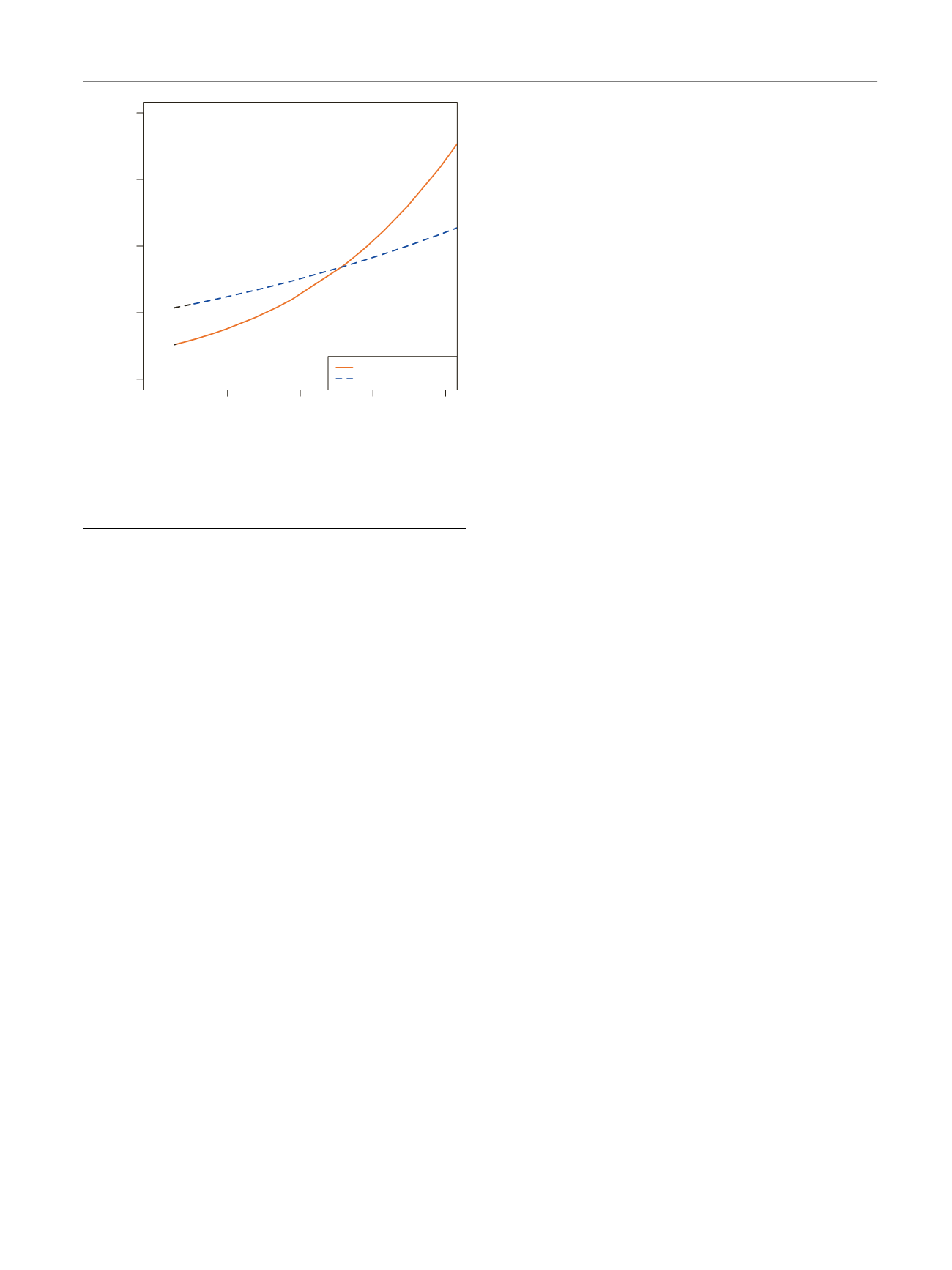
postoperative RT
( Fig. 3 ). Herein, we found that the receipt
of postoperative RT was associated with a survival benefit
only for those patients with a risk of CSM of 30%.
4.
Discussion
Up to 20% of PCa patients experience PSA persistence after
RP, defined as a PSA level 0.1 ng/ml 6 wk following surgery
[4–8]. These individuals have been found to be at increased
risks of disease recurrence and mortality
[4,7–10]. There-
fore, a role for additional cancer therapies such as RT in this
setting has been proposed
[4,12,13,22] .Nevertheless, it
remains unknown whether all patients who fail to achieve
an undetectable PSA level after RP would benefit from
additional treatments to the same extent. Accurate patient
selection is therefore necessary to identify those men who
would most benefit from postoperative RT with regard to
long-term oncologic control, while sparing possible treat-
ment-related side effects among those patients who are
unlikely to progress even in the presence of detectable PSA
after surgery. With this background, we aimed both to
assess the long-term outcomes of men with PSA persistence
after RP, stratified by PCa pathologic features, and to
evaluate the association of postoperative RT with survival
according to the individual risk of CSM using a large multi-
institutional cohort of contemporary patients.
Several results of our study are noteworthy. First, we
demonstrated that the prognosis of men with PSA persis-
tence is not invariably poor. In fact, approximately four out
of five patients did not experience CSM at 10-yr follow-up.
Moreover, we determined that higher pathologic grade
group and the presence of pT3b/pT4 disease were associat-
ed with an increased risk of CSM. Of note, our results are in
line with previous investigations reporting an association
between pathologic characteristics and the risk of mortality
in patients with PSA persistence
[6,11]. Moreover, our
analyses take advantage from the large sample size and
relatively long follow-up, as well as from the inclusion of
patients with detailed data on pathologic characteristics and
administration of postoperative treatments. Importantly, we
then developed a multivariable model to predict individual
patients’ 10-yr CSM risk. Our tool showed a discrimination
accuracy of 67%. We next used this CSM risk prediction to
test the impact of increasing PSA levels at 6–8 wk after RP on
the risk of dying from PCa. Interestingly, we provide what is
to our knowledge the first report that increasing postopera-
tive PSA levels are associated with the risk of dying from PCa
exclusively in men with a CSM risk of
>
10%. This was
particularly evident when the PSA levels increased from
0.1 to 1.4 ng/ml. On the contrary, the slight improvement in
the 10-yr CSM-free survival rates observed when the
postoperative PSA levels increased from 1.4 to 2 ng/ml
might be related to the relatively small number of patients
with a CSM risk of
>
10% and high postoperative PSA levels.
Of note, in men with more aggressive PCa pathology at RP, a
detectable PSA at 6–8 wk might reflect the presence of
persistent malignant prostatic cells either locally or in
distant sites. Although novel imaging modalities such as
prostate-specific membrane antigen positron emission
tomography/computed tomography scan are characterized
by relatively high detection rates in men with biochemical
recurrence even at low PSA levels and might theoretically
discriminate which of these patients harbored persistent
local or distant disease
[23] ,their role in the PSA persistence
setting still needs to be clarified
[2]. Similarly, none of the
available studies on genomic classifiers addressed their
impact in the identification of menwith PSA persistence who
should receive postoperative RT
[24] .As such, our model
based on pathologic characteristics might help clinicians in
identifying patients more likely to have a local recurrence
and who, therefore, would benefit from local salvage
therapies. Of note, we showed that maximizing local
disease control with RT was beneficial in men with a CSM
risk of
>
30%. Given the limited number of patients and
events in higher risk ranges, we were unable to test the
role of postoperative RT in men with even higher risks
of CSM. Conversely, in men with less aggressive disease
(ie, organ confined disease and pathologic grade groups
1–3), PSA level did not impact cancer-specific survival. In
these cases, PSA persistence might be a proxy of benign
prostatic tissue left behind during RP, and the impact of
competing causes of death would thereby be more
pronounced. Further, these patients did not demonstrate a
benefit from postoperative RT.
While data from several previous studies have supported
a benefit for postoperative RT in patients with PSA
persistence
[4,12,13,22], no study to date has tested the
effect of RT according to individual patient profile in this
group of men. Such investigation is highly relevant, as it has
also been shown that a subset of patients with PSA
persistence never experience recurrence. For example,
Rogers et al
[11]demonstrated that more than one out of
five men with PSA persistence who did not receive any
[(Fig._3)TD$FIG]
0.0
0.1
0.2
0.3
0.4
0.0
0.1
0.2
0.3
0.4
Predicted 10-yr CSM
Observed 10-yr CSM
No postoperative RT
Postoperative RT
Fig. 3 – Cancer-specific mortality (CSM) rate plotted against nomogram-
predicted probability of CSM at 10 yr after radical prostatectomy.
Dashed red line indicates postoperative radiotherapy (RT). Solid red line
indicates no postoperative RT.
E U R O P E A N U R O L O G Y 7 2 ( 2 0 1 7 ) 9 1 0 – 9 1 7
915
















