
(Clinical Trial[ptyp] AND (‘‘1998/01/01’’[PDAT]: ‘‘3000/12/
31’’[PDAT]) AND ‘‘humans’’[MeSH Terms] AND ‘‘male’’
[MeSH Terms]). The search, which accrued data from
January 1, 1998 up to October 31, 2016, was restricted to
placebo controlled RCT, which used IIEF for outcome
evaluation.
2.3.
Study selection
All RCTs comparing the effect of TTh versus placebo and
using IIEF as the possible main or secondary outcome were
included in the analyses. Although different other self-
reported inventories have been published and validated for
the evaluation of sexual function
[20] ,the IIEF questionnaire
is the only one which allows for the evaluation of minimal
clinically important differences
[21] .Observational studies
or studies not using IIEF as primary outcome were excluded
from the analysis
( Fig. 1 ). Studies using androgens other
than TTh as well as studies with simultaneous treatment
with other hormones and drugs were also excluded, unless
there was a clearly defined treatment arm that received only
T treatment. In addition, since phosphodiesterase type
5 inhibitors (PDE5is) have been reported to play a possible
positive influence on T levels, trials evaluating the effect of
TTh as an add-on to PDE5is were excluded from the analysis
( Fig. 1). The search was restricted to English-language
articles and human studies. The identification of relevant
studies was performed independently by two of the authors
(G.C. and G.R.), and conflicts resolved by a third investigator
(M.M). Items for the assessment of quality reported in the
Cochrane handbook were used for the evaluation of RCTs
included in the study
[22].
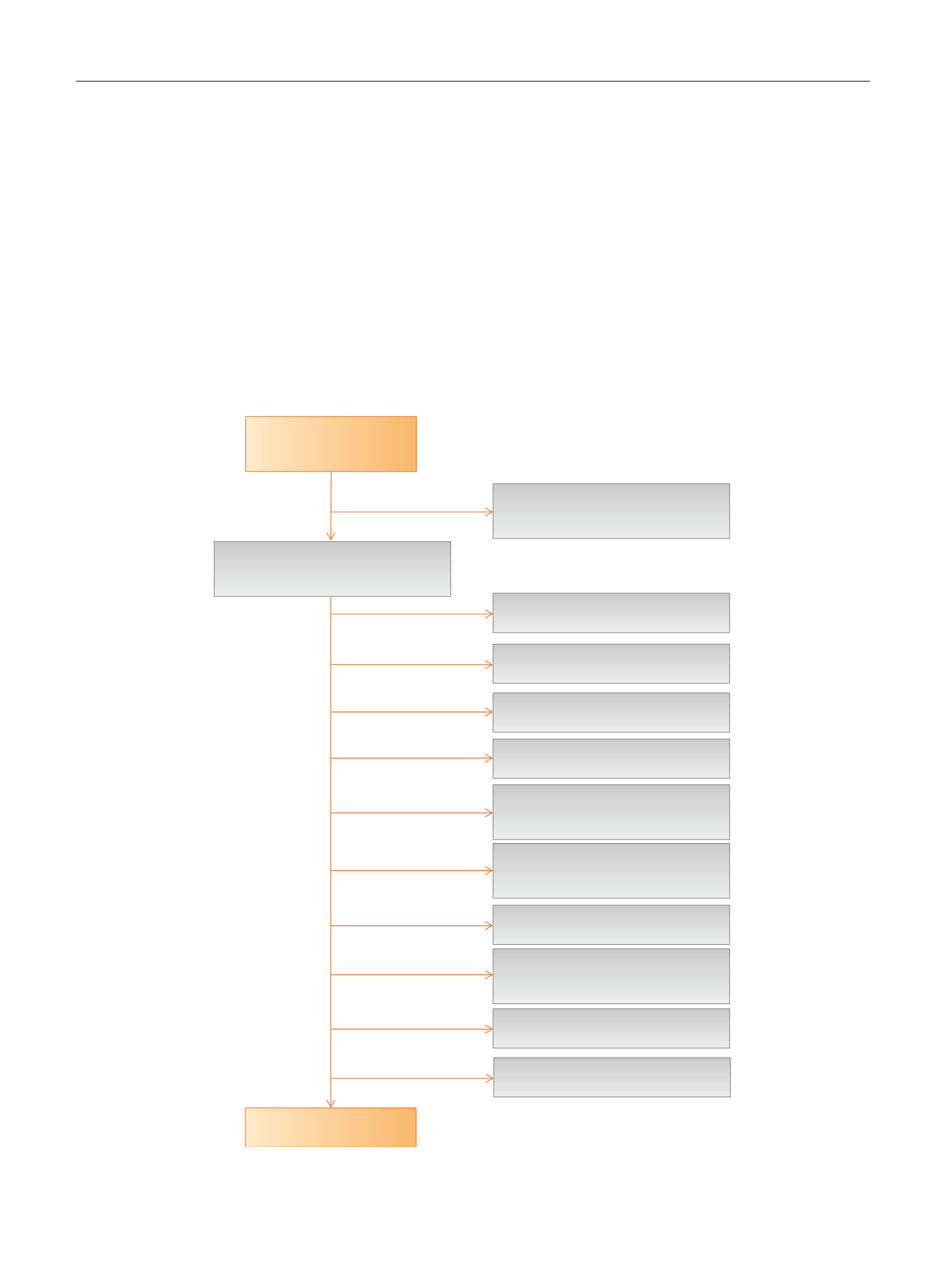
[(Fig._1)TD$FIG]
Published studies
Medline/Embase/Cochrane
search
N
= 284
Citations clearly not relevant based on
abstract and/or title
N
= 141
Potentially relevant articles retrieved for
detailed examination
N
= 143
Retrieved
N
= 14
Reviews
N
= 1
No testosterone replacement therapy
N
= 48
No RCT design
N
= 21
Testosterone replacement therapy
combined with different therapies
N
= 11
Only baseline data from a RCT before
final publication
N
= 1
No data on sexual function
N
= 8
ED evaluated with instruments other than
IIEF
N
= 18
Specific populations
N
= 9
No placebo as comparator
N
= 10
Studies on women
N
= 2
Fig. 1 – Trial flow diagram.
ED = erectile dysfunction; IIEF = International Index of Erectile Function; RCT = randomized clinical trial.
E U R O P E A N U R O L O G Y 7 2 ( 2 0 1 7 ) 1 0 0 0 – 1 0 1 1
1002
















