
and, using the UCLA-PCI questionnaire, reported statistically
worse sexual function for patients in the RP arm. Namiki et al
[20]also reported significantly better urinary function
scores for patients treated with BT. Another small study
[15]reported that at 12 mo sexual function was impaired
significantly in patients after non-NSRP but not after NSRP
when compared with patients treated with BT. In the largest
prospective study
[19] ,authors compared patients treated
with RP, BT, and cryotherapy. Statistical comparison for QoL
outcomes between RP and BT cannot be made (as
cryotherapy is not included in this review); nevertheless,
at 3 yr of follow-up, BT patients tended to have better sexual
and urinary scores.
Only two studies
[16,22]compared QoL after BT and
EBRT using the EPIC questionnaire. Pinkawa et al
[22]reported that BT was associated with statistically signifi-
cantly higher urinary toxicity at 16 mo. Evans et al
[16]reported similar results; however, SBRT was associated
with lower bowel toxicity than BT at 2 yr.
3.5.
Discussion
3.5.1.
Principal findings
The current review synthesises the existing evidence
regarding cancer-specific QoL outcomes of competitive
treatments for clinically localised PCa. QoL is an important
end point in PCa treatment, and recently the COMPACTERS
study group, which developed a core outcome set for trials
of effectiveness, identified QoL as an outcome which should
be measured in all clinical trials of localised PCa
[31] .Out-
comes were measured by PROMs, and the three most used
studies among the those included were EPIC, UCLA-PCI, and
EORTC QLQ-C30.
The ProtecT trial
[12]provides level 1 evidence for the
different effects of PCa treatments on disease-specific QoL
.
No difference was found among treatment modalities in
global QoL at 5 yr. However, surgery had a negative effect on
urinary continence and sexual function, EBRT was associat-
ed with a negative effect in bowel function which was more
intense in the 1st year after treatment, while active
monitoring had the lowest impact on disease-specific QoL
at 6 yr. PCOS 5-yr results
[26]confirm that men who
underwent RP had a higher prevalence of urinary inconti-
nence and erectile dysfunction, while those treated with
EBRT had a higher prevalence of bowel dysfunction. Results
from ProtecT trial are also comparable with the findings of
the Prostate Cancer Intervention Versus Observation Trial
[32]as authors reported that at 2 yr, urinary incontinence
and erectile dysfunction were significantly more common
among men who were assigned to RP when compared with
men managed with observation.
Most other observational studies provide similar, con-
sistent, intermediate-term results for RP and EBRT. Howev-
er, in a recently published study
[30], investigators reported
that although EBRT was associated with a negative effect in
bowel function, the difference in bowel domain score was
below the threshold for clinical significance 12 mo after
treatment. As 81% of patients in the EBRT arm of the study
received IMRT, these data suggest that the risk of side
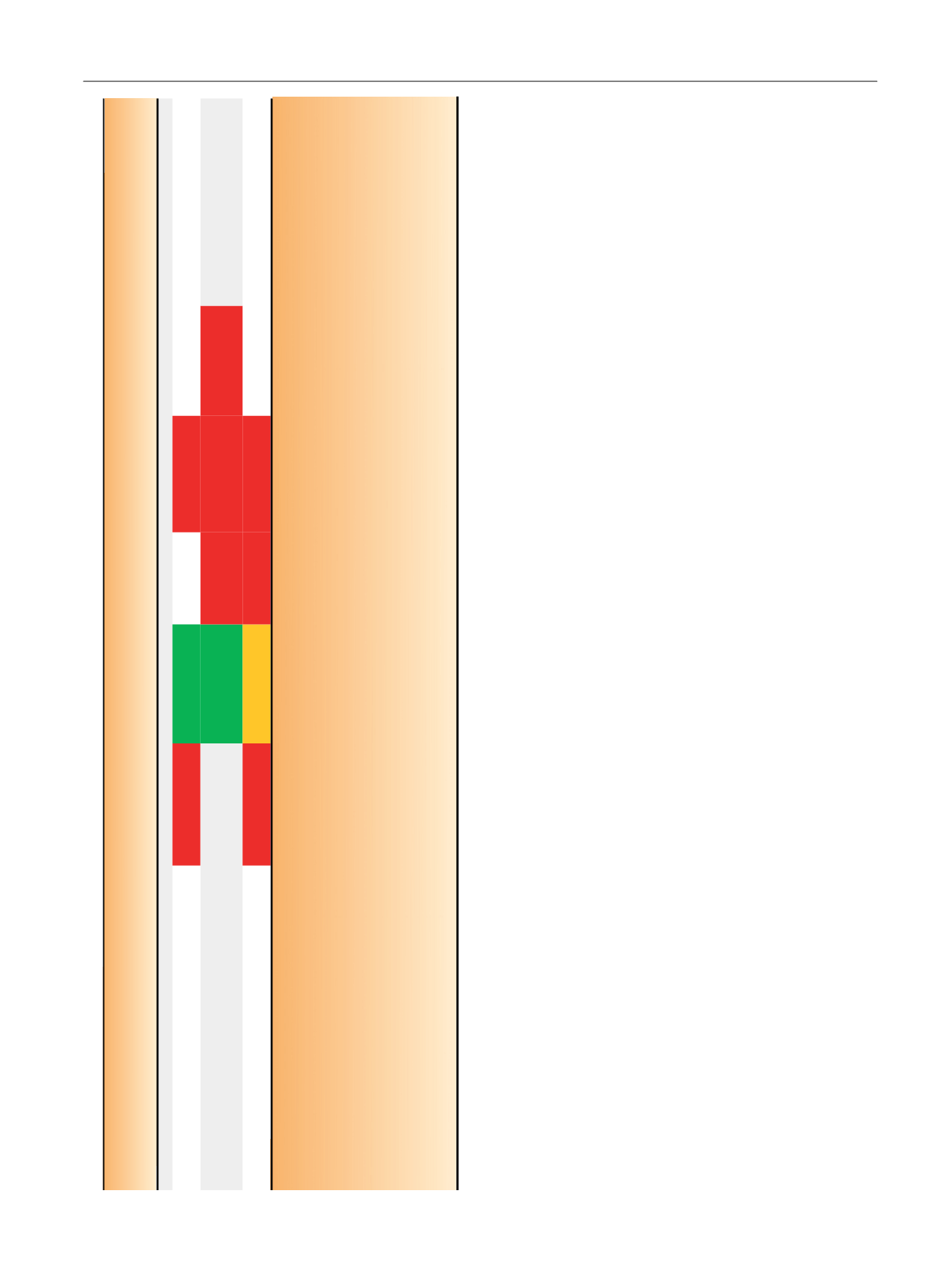
Table 4 (
Continued
)
Author (year)
(longest follow-up time)
Questionnaire used
Intervention
N
at baseline
Reported results
a (latest follow-up data of each study)
Studies that report change in QoL score within groups
Sanda
(2008)
[27]
(24 mo)
Radical
prostatectomy
603
Urinary Incontinence
bUrinary obstructive–
irritative
bBowel function Sexual function
Hormonal function
EPIC
EBRT
292
Urinary Incontinence
bUrinary obstructive–
irritative
bBowel function Sexual function
(only for EBRT
plus NHT)
Hormonal function
(only for EBRT
plus NHT)
Brachytherapy
306
Urinary Incontinence
bUrinary obstructive–
irritative
bBowel function Sexual function
Hormonal function
EBRT = external beam radiotherapy; EPIC = Expanded Prostate Cancer Index Composite; IMRT = intensity-modulated radiotherapy; N = number of patients; NHT = neoadjuvant hormone therapy; NSRP = nerve-sparing
radical prostatectomy; PCOS = Prostate Cancer Outcomes Study; QoL = quality of life; RP = radical prostatectomy; SBRT = stereotactic body radiotherapy; UCLA-PCI = University of California, Los Angeles Prostate Cancer
Index.
For studies comparing interventions, the red colour indicates statistically significant deterioration between a treatment group and the reference group of the study. For studies that report changes in QoL within groups, it
indicates statistically significant deterioration of QoL score from baseline. Orange colour also indicates statistically significant deterioration; however, the difference between groups or within groups does not exceed the
minimally important difference for clinical significance, as defined by authors. This is applicable only for studies reporting minimally important difference for clinical significance. For studies comparing interventions green
colour indicates statistically significant improvement between a treatment group and the reference group of the study. For studies that report changes in QoL within groups, it indicates statistically significant improvement
of QoL score from baseline. No colour indicates no statistically significant difference (for studies comparing interventions, it corresponds to the intervention used as reference and to those that do not show significant
difference with this reference; for studies that report changes in QoL within groups, it shows intervention for which the QoL did not show significant change over time).
a
Malcolm et al’s study
[19]
isnot presented in the table as authors report
p
values for between-group comparisons taking into consideration the cryotherapy arm, which we are not including in our review. Symon et al’s
study
[29]
isalso not presented in the table as authors do not report differences in disease-specific QoL scores among different treatments.
b
In EPIC questionnaire, urinary function items and bother items are combined in the urinary incontinence subscale and the urinary irritation/obstruction subscale.
E U R O P E A N U R O L O G Y 7 2 ( 2 0 1 7 ) 8 6 9 – 8 8 5
881
















