
prospective, randomised (2:1), double-blind, vehicle con-
trolled, multicentre phase 2b evaluated 92 patients eligible
for inclusion with regard to safety and efficacy of a single
transperineal injection
[39]. Treatment with PRX302
resulted in a 9-point improvement in IPSS that was stable
throughout the follow-up period of 12 mo. Mean change
from baseline (PRX302 minus placebo) was 3.3 points after
3 mo (
p
= 0.04) and 2.8 points at 12 mo (
p
>
0.05). Impact on
Q
max
showed an increase of 3 ml/s that was stable during
the study period of 12 mo. No significant effect was
observed for the reduction of prostate volume or postvoid
residual urine volume. No compromising effect on erectile
dysfunction was reported. Adverse events were mild to
moderate and transient in nature. Recently, the sponsor
(Sophiris Bio Corp, CA, USA) announced that a prospective,
randomised, double-blind, placebo-controlled phase 3 trial
was successful to meet primary endpoint
( http://investor. sophirisbio.com/releasedetail.cfm?ReleaseID=969508). Af-
ter 12 mo, a reduction in IPSS of 7.6 points after transrectal
injection of PRX302 was statistically superior to an
improvement of 6.58 points in the vehicle control
(
p
= 0.043). This encouraging result warrants further
randomized controlled trials needed to define the role of
the intraprostatic injection of PRX302 in the spectrum of
minimally invasive treatment modalities for male LUTS due
to BPE.
[12_TD$DIFF]
3.5.
Mechanical devices
Mechanical devices to establish and preserve urethral
patency have been introduced as temporary or permanent
treatment options for bladder outlet obstruction secondary
to BPE as an alternative to indwelling catheters or for
patients unfit for surgery. Over the years the concept of
prostatic stents attracted renewed interest, as technical
modifications were constantly developed to optimize
relevant issues like a reduction of migration rate, biocom-
patibility, encrustation, misplacement, and perineal pain
after implantation. However, it is the advent of novel
mechanical concepts to deobstructing the prostatic lumen
that seem to prove their worth as minimally invasive
approach.
[13_TD$DIFF]
3.6.
TIND
[14_TD$DIFF]
3.6.1.
Basic principle
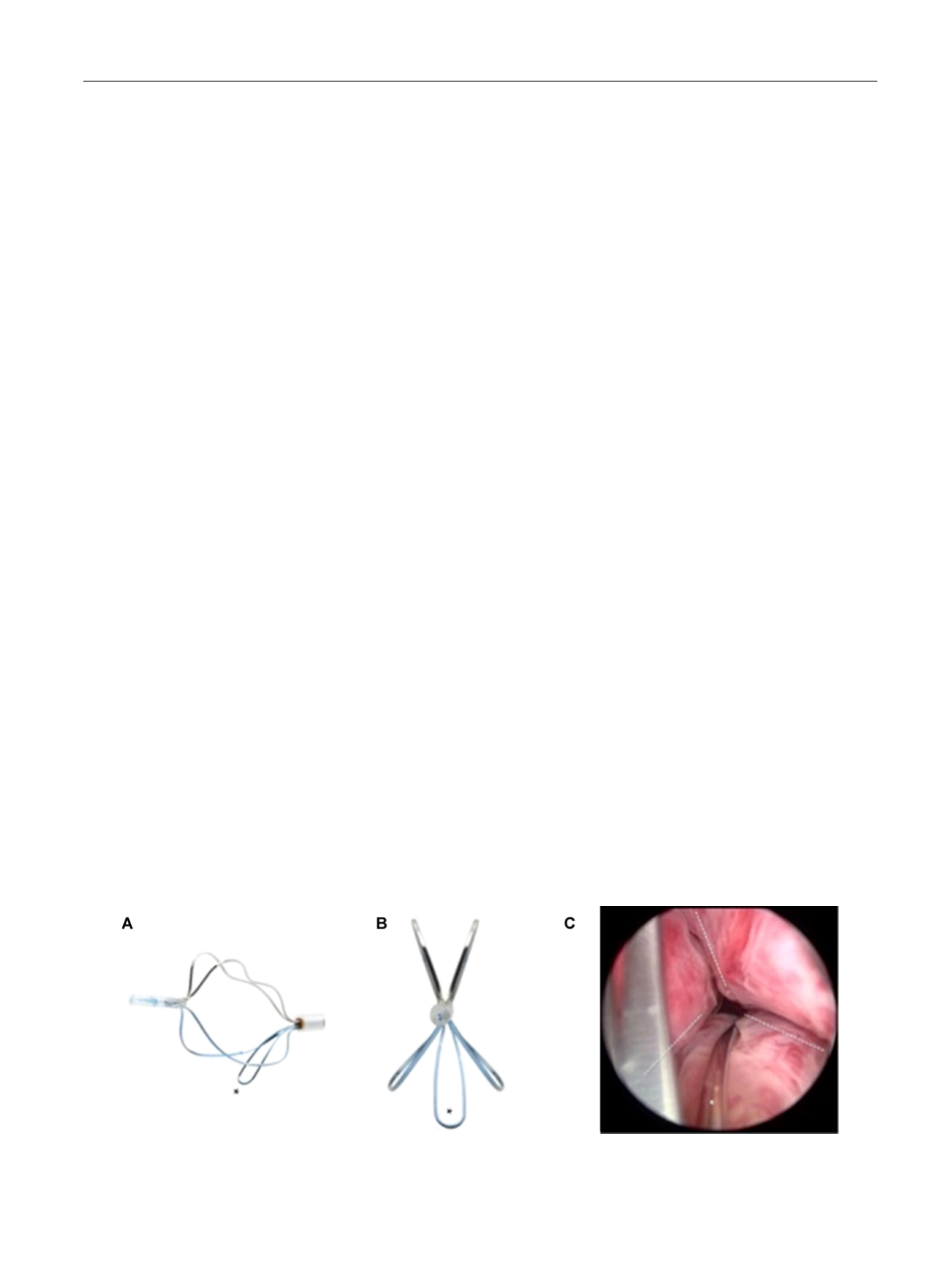
TIND (Medi-Tate; Medi-Tate Ltd., Or Akiva, Israel) is an
emerging device designed to remodel the bladder neck and
the prostatic urethra in an unprecedented way
( Fig. 1). The
TIND is composed of elongated struts and an anchoring
leaflet, all made of nitinol. Under cystoscopic guidance the
device is preloaded on a dedicated delivery system and
advanced through a standard 22-F cystoscope sheath in an
up-folded configuration. The dimensions of this tool (total
length: 50 mm, outer diameter: 33 mm) were designed to
cover the complete prostatic urethra, ranging from bladder
neck to proximal to the external urinary sphincter. Under
direct visualisation the TIND is deployed inside the bladder
in expanded configuration. Anchoring the leaflet slide at the
6 o’clock position distal to the bladder neck ensures precise
and safe positioning within the bladder neck and the
prostatic urethral lumen. The intended mode of action is to
compress obstructive tissue by the expanded device, whose
struts will exert radial force leading to ischaemic necrosis in
defined areas of interest. The TIND is left in position for 5 d,
time enough to create prostatic incisions anteriorly, at the
5 o’clock and 7 o’clock positions. The resulting incisions may
be similar to a Turner Warwick incision. In an outpatient
setting the device is safely removed by standard urethro-
scopy.
[15_TD$DIFF]
3.6.2.
Clinical outcome
A single-arm, prospective study on 32 patients was
conducted to evaluate feasibility and safety of the
procedure
[40]. All participants were treated with light
sedation, mean operative time was 5.8 min and after the
20th procedure patients were discharged on the same day of
intervention. This first study reported that the device was
well tolerated by all patients. Overall, four postoperative
complications (12.5%) such as prostatic abscess, urinary
retention, transient incontinence, and urinary tract infec-
tion were recorded. No late complications, adjunctive
reinterventions, or medical therapy were documented at
the 12-mo follow-up. First functional outcomes suggest
efficacy of the technique. After 12 mo, mean changes
[(Fig._1)TD$FIG]
Fig. 1 – Temporary implantable nitinol device (TIND). (A) TIND in its expanded configuration, longitudinal view. (B) TIND in its expanded configuration,
front view. (C) Cystoscopic visualisation: the anchoring leaflet is in its correct position (*) and the incisions are visible (—). Length: 50 mm, width:
33 mm.
E U R O P E A N U R O L O G Y 7 2 ( 2 0 1 7 ) 9 8 6 – 9 9 7
989
















