
Letter to the Editor
Re: Emmanuel S. Antonarakis, Changxue Lu, Brandon
Luber, et al. Clinical Significance of Androgen Receptor
Splice Variant-7 mRNA Detection in Circulating Tumor
Cells of Men with Metastatic Castration-resistant
Prostate Cancer Treated with First- and Second-line
Abiraterone and Enzalutamide. J Clin Oncol
2017;35:2149–56
AR-V7 Testing: What’s in it for the Patient?
We congratulate Antonarakis et al
[1]on their study and for
providing additional important data on AR-V7 in the setting
of castration-resistant prostate cancer (CRPC). By expanding
on their prior work, the authors confirm the negative
prognostic impact of circulating tumor cell (CTC) detection
and CTC-based AR-V7 mRNA detection in patients with
CRPC. The authors state that assessing these biomarkers
may be useful in predicting responses to therapies. On the
basis of their data, they propose a shift from current clinical
practice towards biomarker-based treatment decisions
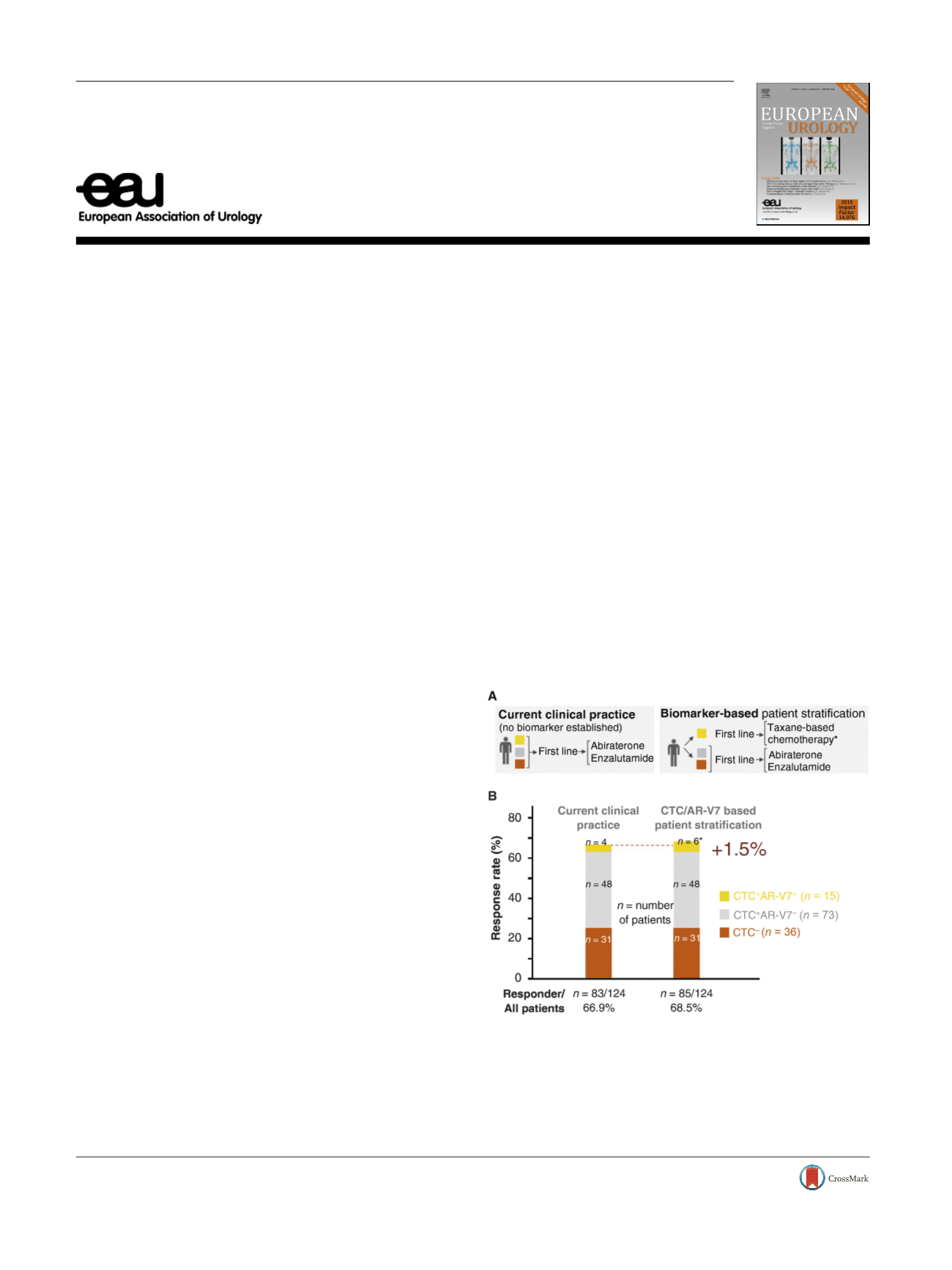
( Fig. 1A). We find the study data in the first-line setting
noteworthy for several reasons.
First, by showing that four of 15 CTC
+
AR-V7
+
patients
(26%) achieved prostate-specific antigen decreases to
>
50%
from baseline, the authors resolve a prior controversy
regarding these unexpected responses to abiraterone or
enzalutamide
[2–4]. We previously reported a 20% response
rate among CTC
+
AR-V7
+
patients on abiraterone or
enzalutamide
[3]. Antonarakis et al
[1]used appropriate
clinical trial criteria to confirm these unexpected responses.
Thus, the AR-V7 status in CTCs cannot entirely predict non-
response, and CTC
+
AR-V7
+
patients should not be
systematically denied abiraterone or enzalutamide treat-
ment
[3] ,especially given the response rates to alternative
treatment options
[5] .Second, a major as-yet unanswered question that must
be addressed before a biomarker can be translated to the
clinic is the ability of CTC AR-V7 testing to predict responses
or non-responses to treatment. Importantly, prospective
biomarker-stratified trials are currently missing. In their
study, Antonarakis et al
[1]did not use biomarker-based
treatment stratification of patients, and all patients enrolled
received abiraterone or enzalutamide on the basis of a
clinical decision. The data thereby allow assessment of
overall response rates in current clinical practice
( Fig. 1 B).
The overall response rate to abiraterone or enzalutamide in
the first-line setting was 66.9% (
n
= 83 responders from
124 patients). These data allow estimation of the overall
benefit from implementation of CTC AR-V7 testing and
treatment stratification in the first-line setting. As pointed
out in several publications
[5–7], the key therapeutic
alternative for patients when identified as CTC
+
AR-V7
+
is
taxane-based chemotherapy, whereas treatment decisions
for other subgroups remain the same. To estimate the
response rates to chemotherapy in the specific subgroup of
AR-V7
+
patients, we used recent meta-review data that
showed a response rate of 35.8% (
n
= 19/53)
[8]. Compari-
son of these response rates in the AR-V7
+
setting between
abiraterone or enzalutamide (26.6%;
n
= 4/15) and taxane-
based chemotherapy (35.8%;
n
= 19/53) did not reveal a
significant difference (
p
= 0.76, Fisher’s exact test). When
applying the 35.8% response rate to taxanes to the 15 AR-
V7
+
patients in the Antonarakis et al study
[1] ,we calculated
the theoretical number of responders as 5.37 patients
E U R O P E A N U R O L O G Y 7 2 ( 2 0 1 7 ) e 1 6 8 – e 1 6 9available at
www.scienced irect.comjournal homepage:
www.europeanurology.com[(Fig._1)TD$FIG]
Fig. 1 – AR-V7 testing: what’s in it for the patient? (A) Currently, no
predictive biomarker has been established in castration-resistant
prostate cancer. (B) Based on the data reported by Antonarakis et al
[1] ,the diagram depicts the estimated overall benefit from biomarker-
based patient stratification. The asterisk * indicates the predicted
number of CTC
+
AR-V7
+
responders to taxane-based chemotherapy on
the basis of recent meta-review data
[8] .Note that prospective
biomarker-stratified trial data are currently missing. CTC = circulating
tumor cell.
http://dx.doi.org/10.1016/j.eururo.2017.06.0310302-2838/
#
2017 European Association of Urology. Published by Elsevier B.V. All rights reserved.
















