
Research Letters
In
fl
uence of Prior Tyrosine Kinase Inhibitor on Survival for
Patients with Metastatic Renal Cell Carcinoma Treated
with Nivolumab or Cabozantinib: Data from a
Literature-based Meta-analysis
Giandomenico Roviello
a , b , * , Daniele Generali b , cFirst-line treatment of metastatic renal cell carcinoma still
involves small-molecule tyrosine kinase inhibitors (TKIs)
such as sunitinib and pazopanib, which mainly target the
vascular endothelial growth factor receptor (VEGFR)
[1]. It
has recently been shown that two agents improve survival
in second-line treatment. In the METEOR trial
[2], cabo-
zantinib, a multikinase inhibitor targeting the VEGFR and
other pathways including MET, RET and AXL, and in the
CheckMate 025 study
[3] ,nivolumab, an immunotherapeu-
tic agent that inhibits the T-cell checkpoint regulator PD-1,
were compared to everolimus and showed a survival
advantage. However the most effective sequence after
first-line TKI treatment is still unknown, so there is a need to
identify factors that predict the response to nivolumab and
cabozantinib. The aimof this letter is to focus on the survival
of patients treated with nivolumab and cabozantinib
according to prior first-line TKI. We used data from
CheckMate 025 and METEOR for a subsequent subgroup
analysis
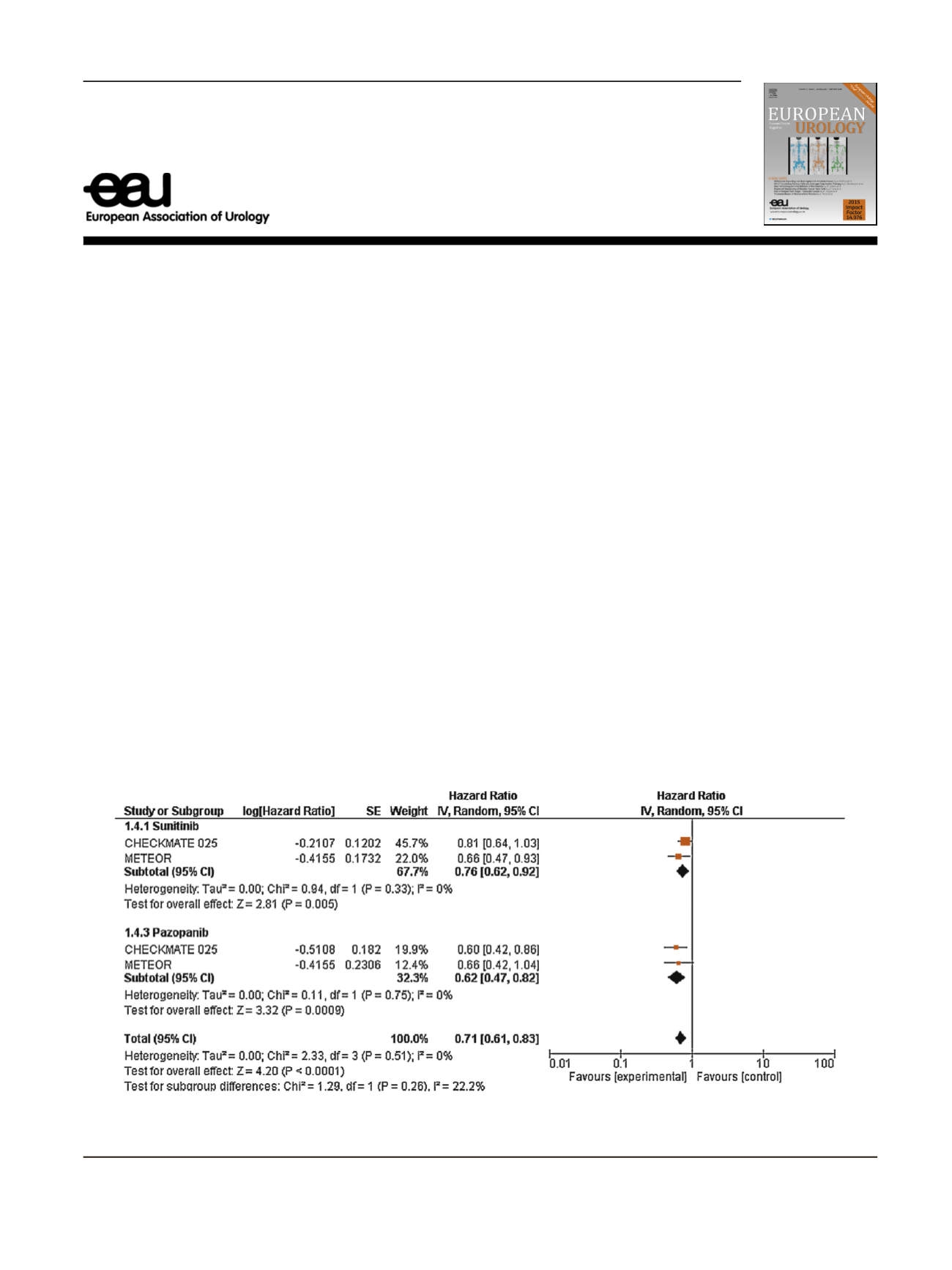
( Table 1)
[4,5]. A pooled analysis according to prior
TKI treatment revealed that survival was significantly
improved to a greater extent after prior treatment with
pazopanib (hazard ratio [HR] 0.62, 95% confidence interval
[CI] 0.47
–
0.82;
p
= 0.0009) when comparison to sunitinib
(HR 0.76, 95% CI 0.62
–
0.92;
p =
0.005;
Fig. 1 ).
Athough a limitation of this analysis is that literature
data were used rather than a meta-analysis of data for
individual patients, so that definitive conclusions need to be
considered carefully, our data show that cabozantinib and
E U R O P E A N U R O L O GY 7 2 ( 2 0 17 ) 10 2 7 – 10 2 9ava ilable at
www.sciencedirect.comjournal homepage:
www.eu ropeanurology.com[(Fig._1)TD$FIG]
Fig. 1
–
Subgroup analysis for overall survival among patients receiving nivolumab and cabozantinib according to prior sunitinib or pazopanib.
CI = confidence interval.
0302-2838/© 2017 European Association of Urology. Published by Elsevier B.V. All rights reserved.
















